TriStem’s Technology
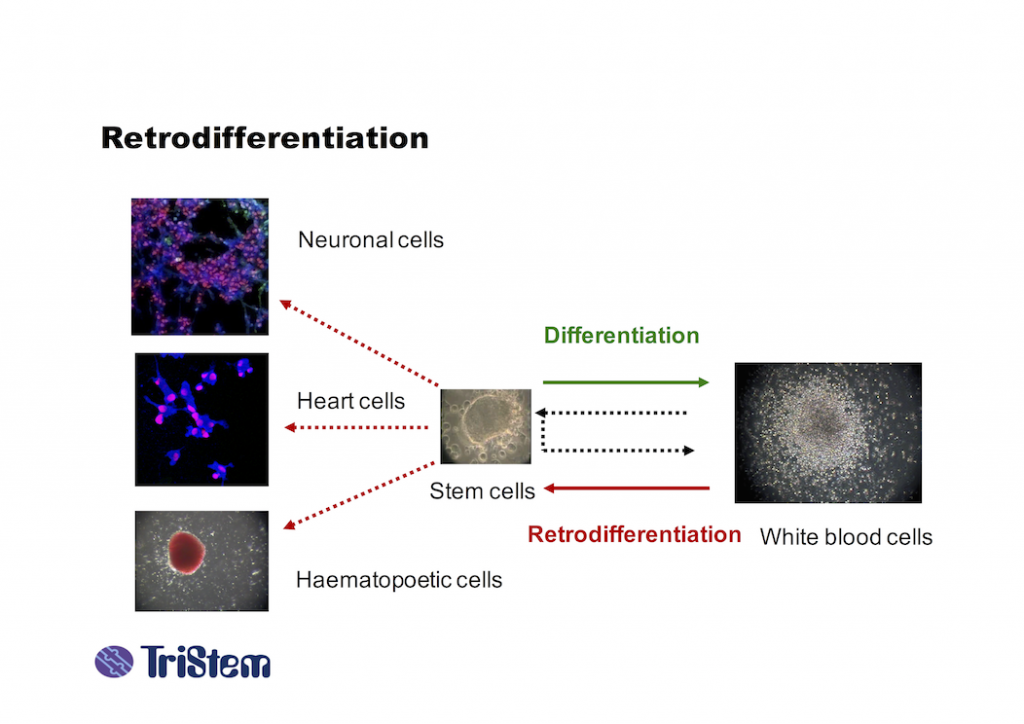
TriStem owns a patented process whereby fully differentiated human cells can be retrodifferentiated into stem cells with the potential to re-differentiate into any other functional cell type, in other words they are multipotent.
This research underlying the procedure has been published in a peer-reviewed journal which can be found here.
TriStem’s technology may be a significant improvement over existing stem cell transplantation for the following key reasons:
- the patient is their own donor, thus negating the need for a donor and immunosuppressant therapy post transplant to avoid rejection, reducing procedure and drug costs;
- the number of stem cells that TriStem is able to generate is greater than is possible by conventional means. Up to 16 billion stem cells can be generated from a single patient in a single procedure over 5-24 hours, compared to 70 million by repeated harvesting over 5-6 weeks, and
- no embryonic or foetal tissues are used in the procedure and there are therefore no ethical issues.
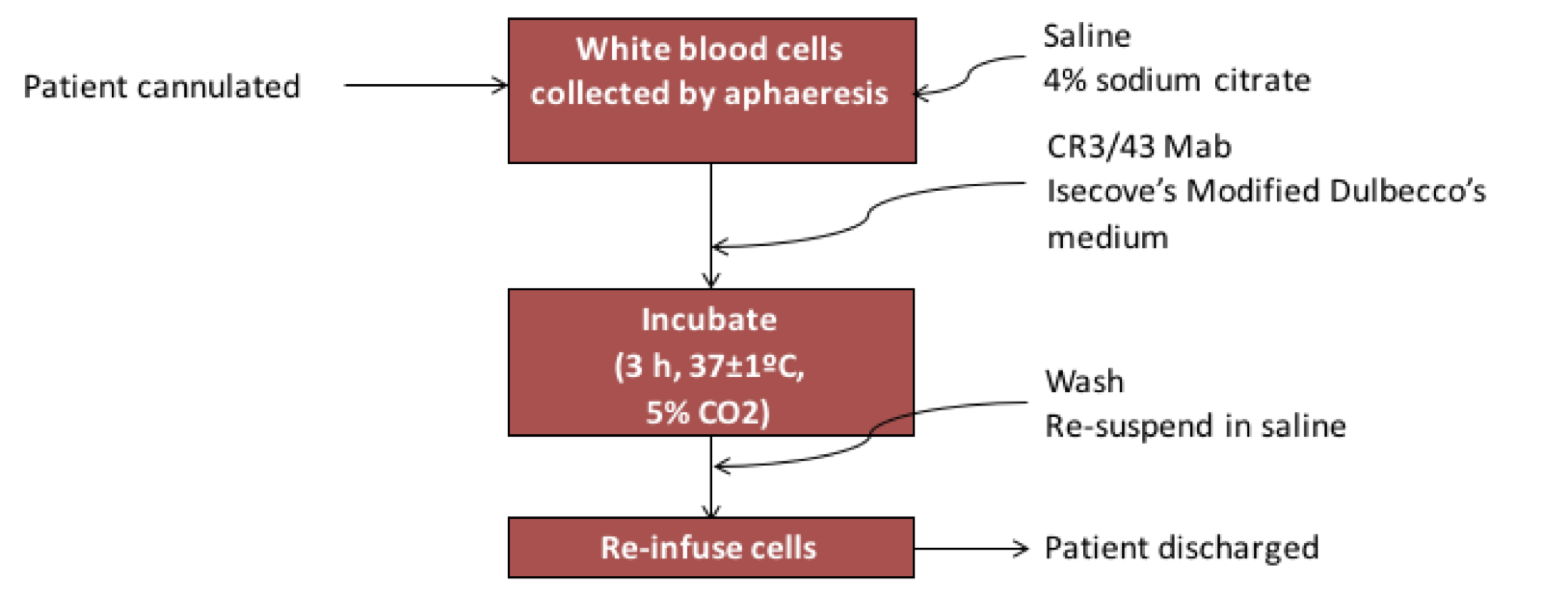
The procedure is non-surgical and uses equipment and techniques that are in routine use in major hospitals world-wide. The procedure can be summarised as follows:
There are several sources for the preparation of stem cells. The following table compares the risks and benefits of the major sources, comparing the features, benefits and risks of each to TriStem’s patented Retrodifferentiation technology.
“TriStem’s” Retrodifferentiation |
Bone marrow harvest |
Peripheral blood |
Umbilical cord stem cells |
Embryonic stem cells (ESC) |
Mesenchymal stem cells (MSC) |
Induced pluripotent stem cells (iPS) |
|
Number of stem cells produced |
Unlimited | Small | Small | Small | Theoretically unlimited | Small | Theoretically unlimited |
Time taken |
Several hours | Weeks | Weeks | Hours | Weeks | Weeks | Unknown |
Pre-treatment required |
None | Yes | Yes | None | None | Yes | None |
Side effects |
None | Severe bruising to pelvis Side effects of GCSF therapy |
Side effects of GCSF therapy | None | Tumour formation (teratogenesis) | Side effects of GCSF therapy | Not known: in theory none |
Hospitalisation for donor |
None | 1 or 2 days hospitalisation per harvest | None | None | None | 1 or 2 days hospitalisation per harvest | None |
Post-treatment required |
None | Yes | Yes | Yes | None | None | Not known: in theory none |
Cost |
$ | $$$ | $$ | $$ | $$ | $$$ | Unknown |
Ethical issues |
None | None | None | None | Significant | None | None |
Tissue match required |
No (autologous); Yes (allogeneic) |
Yes | Yes | Yes | No | Yes | Not known: in theory no |
Regulatory issues |
None | None | None | None | Genetic Modification | None | Genetic Modification |
Use |
Broad patented disease applications | Blood disorders, severe burns, corneal damage | Blood disorders | Blood disorders | Several diseases under clinical investigation | Musculoskeletal diseases | Not yet in clinical use |
Clinical experience |
yes | yes | yes | yes | yes | yes | no |